Bone Healing Therapy
Bone healing therapy is a safe, non-surgical treatment to activate the body's natural healing process that may be impaired.
BONE HEALING SOLUTIONS
Proven Effective Therapy to Stimulate Fracture Repair
 In some patients who have a fracture, the bone(s) may not mend properly, and a nonunion results. To help overcome this healing challenge, doctors prescribe bone growth therapy, commonly known as bone growth stimulation.
In some patients who have a fracture, the bone(s) may not mend properly, and a nonunion results. To help overcome this healing challenge, doctors prescribe bone growth therapy, commonly known as bone growth stimulation.
Orthofix Bone Growth Therapy devices have been prescribed since the late 1980s to provide adequate stability and allow for early functional recovery thereby improving patients’ quality of life. Bone growth therapy is a safe, non-surgical treatment to activate the body’s natural healing process that may be impaired.
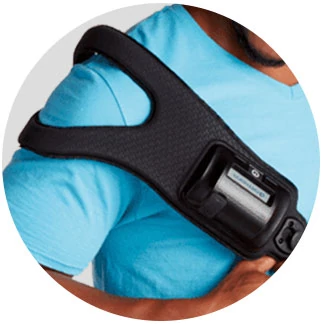
The PhysioStim™ device provides a safe, noninvasive option for treating fractures that are difficult to heal. With high clinical success rates, the PhysioStim device provides 360 degree of treatment coverage around the fracture site. The device assists in fracture healing by delivering a pulsed electromagnetic field (PEMF) signal to the targeted fracture site.
PhysioStim Areas of Treatment
Why Do Physicians Prescribe a PhysioStim Device?

High clinical success rates.1,2,3

PEMF signal covers 360 degrees around the fracture site.4

Ease of placement enables consistent treatment of the fracture site.
Penetrates evenly across tissue, bone and fixation.5

AccelStim Helps Patients Accelerate Fracture Recovery for Indicated Fresh Fractures
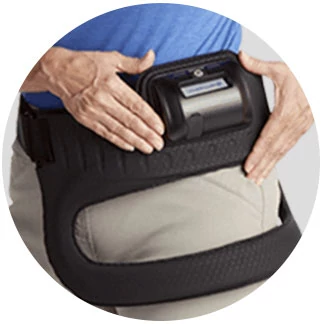
The AccelStim™ device transmits a low-intensity ultrasound signal to the fracture site through coupling gel, with little or no sensation felt by the patient during the treatment.6-9 Low-intensity pulsed ultrasound has been shown in invitro and in vivo studies to stimulate cells to produce growth factors and proteins that are important to bone healing.
Why Do Physicians Prescribe an AccelStim Device?
The AccelStim bone growth therapy device is FDA approved to be used for the treatment of nonunion fractures and to accelerate the healing of indicated fresh fractures.9 Please refer to the instruction manual for complete prescribing information.

86% clinical success rates for nonunion fractures.6
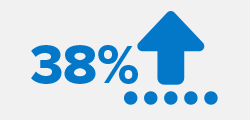
38% acceleration in healing for indicated fresh fractures.7
LIPUS direct signal to the fracture site.

20 minutes daily treatment time.
Fracture Healing with Bone Growth Stimulators
Fracture healing is a complicated metabolic process and is dependent on many factors. Healing fractures may be delayed or impaired if these factors are inadequate or interrupted. Most fractures heal without any complications. However, some patients develop nonunions and/or delayed unions, and are not healing on their own.
There are various ways to deal with nonunion, including surgery, internal or external fixation, bone grafting, or the use of biologic bone substitutes. The least invasive and only non-surgical option is a durable medical equipment device called a bone growth stimulator. The most commonly used bone stimulation devices send more energy to the healing bone surface through either pulsed electromagnetic or ultrasound waves, which helps the bone heal more quickly.
What is a Bone Stimulator Used For?
Bone growth stimulator devices are used as a non-surgical treatment option to assist in healing bone fractures throughout the body. The most common scenarios in which bone stimulators are prescribed are when a patient presents with a slow healing, nonunion fracture that might have included surgery to repair. When a patient is identified with a bone healing issue, bone stimulator devices may be prescribed.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Services at 1-800-535-4492.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
The AccelStim™ device is indicated for the non-invasive treatment of established nonunions excluding skull and vertebra, and for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed, or Grade I open tibial diaphysis fractures in skeletally mature adult individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
The PhysioStim™ device is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated. A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
References:
- PMA P850007. February 1986.
- Garland DE, Moses B, Salver W. Fracture healing: Long-term follow-up of fracture nonunions treated with PEMFs. Contemp Orthop. 1991;22(3):295-302. PubMed Abstract
- Orthofix patient registry. PMA P850007/S20. Data on file.
- Data on file. Field mapping analysis conducted by M. Zborowski, Ph.D., Cleveland Clinic.
- Navarro, M., Michiardi, A., Castano, O., & Planell, J.. (2008). Biomaterials in orthopaedics. Journal of the Royal Society Interface, 5(27), 1137-1158.
- Nolte PA, van der Krans A, Patka P, Janssen IMC, Ryaby JP, Albers GHR. Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma. 2001;51(4):693-703.
- Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. J Bone Joint Surg. 1997;79- A(7):961-973.
- Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg. 1994;76- A(1):26-34.
- PMA No: P210035