Bone Healing Therapy
Our bone growth therapy devices enhance fracture healing by stimulating the body’s own natural healing process. These non-invasive portable devices are intended to be used as part of a home treatment program prescribed by a health care professional.
Proven Effective Therapy to Stimulate Fracture Repair
The PhysioStim™ and AccelStim™ devices provide a safe and effective non-surgical treatment to improve fracture healing.

#1 Prescribed Bone Growth Stimulator10-11

Supported by the NASS coverage recommendations12

The PhysioStim device uses a pulsed electromagnetic field (PEMF) signal to induce a low-level electrical field at the fracture site which stimulates bone healing.
Why do Physicians Prescribe the PhysioStim Device?
PEMF Signal covers 360 around the fracture site.4
Ease of placement enables consistent treatment of the fracture site.
Penetrates evenly across tissue, bone, and fixation.5

PhysioStim Areas of Treatment


The PhysioStim device provides a safe noninvasive option for treating fractures that are difficult to heal. With high clinical success rates, the PhysioStim device provides 360° of treatment coverage around the fracture site. The device assisted in fracture healing by delivering a pulsed electromagnetic field (PEMF) signal to the targeted fracture site.

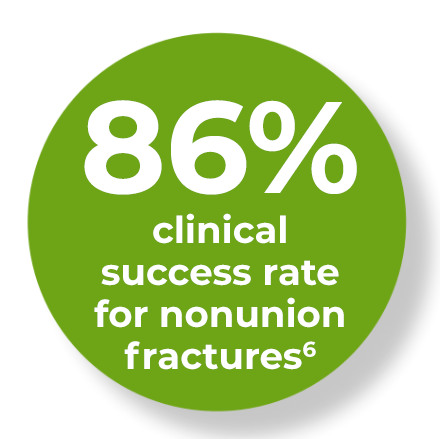
Speed up your fracture recovery and get back to your life faster. The AccelStim device transmits a low intensity ultrasound signal to the fracture site through coupling gel, with little or no sensation felt by the patient during the treatment.6-9
Why do Physicians Prescribe the AccelStim Device?
The AccelStim bone growth therapy device is FDA approved to be used for the treatment of non-union fractures and to accelerate the healing of indicated fresh fractures. Please refer to the instruction manual for complete prescribing information.
LIPUS direct signal to the fracture sites.
20 minute daily treatment time.

Fracture Healing with Bone Growth Stimulators
Fracture healing is a complicated metabolic process and is dependent on many factors. Healing fractures may be delayed or impaired if these factors are inadequate or interrupted. Most fractures heal without any complications. However, some patients develop nonunion and/or delayed unions and are not healing on their own.
There are various ways to deal with nonunion, including surgery, internal or external fixations, bone grafting, or the use of biologic bone substitutes. The least invasive and only non-surgical option is a durable medical equipment device called a bone growth stimulator. The most used bone stimulator devices send more energy to the healing bone surface through either pulsed electromagnetic or ultrasound waves, which help the bone heal more quickly.
What is a Bone Growth Stimulator Used For?
Bone growth stimulator devices are used as a non-surgical treatment option to assist in healing bone fractures throughout the body. The most common scenarios in which bone stimulators are prescribed are when a patient is present with slow healing, nonunion fracture that might have included surgery to repair. When a patient is identified with a bone healing issue, bone stimulator devices may be prescribed.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Care at 1-800-535-4492.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
The AccelStim™ device is indicated for the non-invasive treatment of established nonunions excluding skull and vertebra, and for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed, or Grade I open tibial diaphysis fractures in skeletally mature adult individuals when these fractures are orthopedically managed by closed reduction and cast immobilization.
The PhysioStim™ device is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated. A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
References:
- PMA P850007. February 1986.
- Garland DE, Moses B, Salver W. Fracture healing: Long-term follow-up of fracture nonunions treated with PEMFs. Contemp Orthop. 1991;22(3):295-302. PubMed Abstract
- Orthofix patient registry. PMA P850007/S20. Data on file.
- Data on file. Field mapping analysis conducted by M. Zborowski, Ph.D., Cleveland Clinic.
- Navarro, M., Michiardi, A., Castano, O., & Planell, J.. (2008). Biomaterials in orthopaedics. Journal of the Royal Society Interface, 5(27), 1137-1158.
- Nolte PA, van der Krans A, Patka P, Janssen IMC, Ryaby JP, Albers GHR. Low-intensity pulsed ultrasound in the treatment of nonunions. J Trauma. 2001;51(4):693-703.
- Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. J Bone Joint Surg. 1997;79- A(7):961-973.
- Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg. 1994;76- A(1):26-34.
- PMA No: P210035
- iData Research Inc., U.S. Market for Spinal Implants and VCF (iDATA_USSP22_RPT), iData Research Inc (www.idataresearch.net) 2022
- iData Research Inc., U.S. Market for Orthopedic Trauma Devices (iDATA_USTRA22_RMS), iData Research Inc (www.idataresearch.net) 2022
- Spine.org
*for nonunion fractures 0-3mm
Orthofix products or services referenced herein are trademarks or registered trademarks of Orthofix Medical Inc. and its group of companies. All rights reserved


